
The Lewis dot structure of calcium represents the valence electrons, which in this case, are the two electrons in the 4s orbital. The electron configuration of calcium is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. In the case of calcium (Ca), it has an atomic number of 20, meaning it has 20 electrons. By understanding the electron configuration, we can determine the chemical behavior of an element. The Lewis dot structure provides a simplified way to represent the valence electrons, which are the outermost electrons involved in chemical bonding.
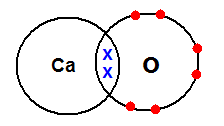
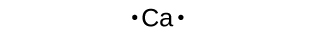
Understanding Electron ConfigurationĮlectron configuration refers to the arrangement of electrons in an atom or ion. Lewis dot structures are essential in understanding the electron configuration, predicting chemical bonding and reactivity, and determining molecular geometry and polarity. Lewis, who introduced the concept in 1916. The Lewis dot structure is a visual representation of the electron arrangement in a molecule or ion. By examining the Lewis dot structures of calcium in various compounds, we can gain insights into the electron arrangement and chemical bonding in these substances. In conclusion, the Lewis dot structure is a useful tool for representing the valence electrons of atoms and understanding the formation of chemical compounds. Iodine is represented by its symbol I with seven dots around it, signifying its seven valence electrons. In the Lewis dot structure of calcium iodide, calcium is represented by its symbol Ca with two dots, indicating the transfer of its valence electrons to iodine. Element Lewis Dot Structure Calcium Ca Iodine I To achieve stability, calcium will transfer its two valence electrons to iodine. Iodine has seven valence electrons, while calcium has two. Lastly, let’s examine the Lewis dot structure of calcium when it combines with iodine to form calcium iodide (CaI2).


 0 kommentar(er)
0 kommentar(er)
